dialogue
studentwise:
Battery Life
by Kate Campbell
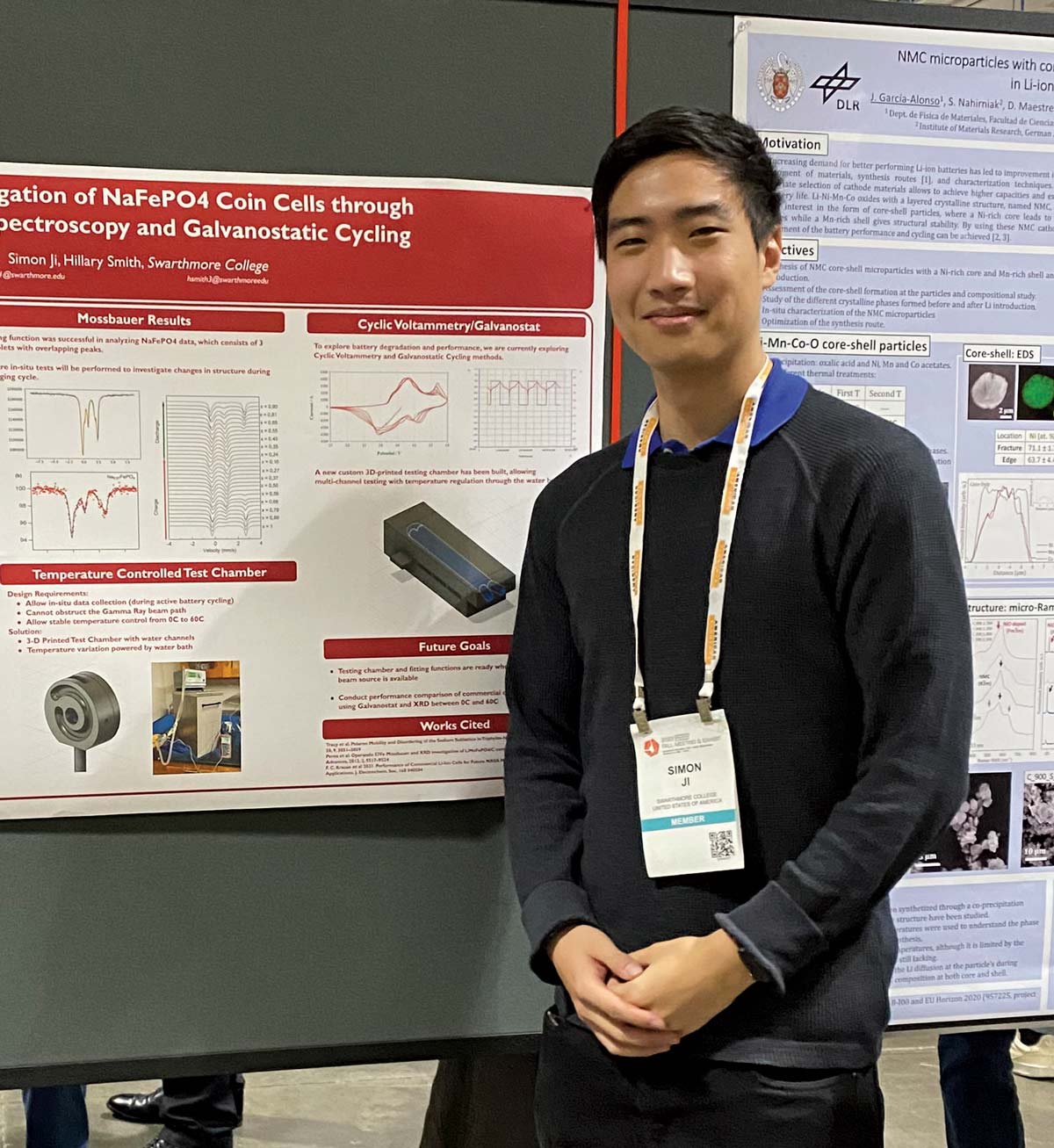
The title of Simon Ji ’23’s research poster is daunting: “In-situ investigation of NaFePO4 Coin Cells through Mossbauer Spectroscopy and Galvanostatic Cycling.” But Ji explains it concisely. “In the hunt for more economical and sustainable battery materials, Sodium Iron Phosphate (NaFePO4) has emerged as a promising candidate due to the abundance of the elements that make up its structure,” Ji says. He hopes his research with Assistant Professor of Physics Hillary Smith will help understand this battery material’s behavior under real-life operating conditions, and provide insights to improve its safety, energy density, and longevity.
courtesy of simon ji ’23
“Batteries made from sodium iron phosphate will be much cheaper and easier to manufacture, leading to less expensive electric cars or grid storage devices for solar and wind [energy],” says Simon Ji ’23. “We hope that these batteries will bring us one step closer to a zero carbon future.”